physical and chemical changes
Physical and Chemical changes differ in that while the former do not change composition, but the latter does. Identifying physical and chemical changes is important because this process allows scientist to understand specific properties.
For the first day of Chemiholics, we delved into distinguishing between physical and chemical experiments. The lineup included:
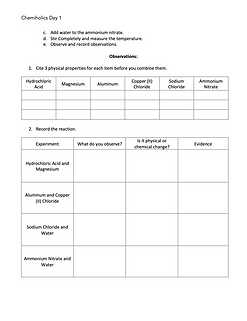
1. Hydrochloric Acid and Magnesium
2. Aluminum and Copper (ii) Sulfate
3. Sodium Chloride and Water
4. Ammonium Nitrate and Water
pre-lab activity


1. hydrochloric acid and magnesium
-
Cut 1 piece of 1cm long magnesium strip and place them into a test tube.
-
Add 5 drops of 2M HCl.
-
Touch the bottom of the test tube.
-
Observe and record the reaction happening.
-
Determine the volume of 5 drops of 2M HCl using a graduated cylinder.
-
Double the volume of drops you determined in step e by adding deionized water.
-
You have just prepared 1M HCl. Repeat steps a-d with the newly prepared HCl solution.
2. Aluminum and copper (ii) sulfate
-
Dissolve 2.0 g of copper (II) chloride with 25.0 ml of distilled water in a beaker.
-
Stir until it's completely dissolved.
-
Measure and cut a piece of aluminum foil (4cm by 4cm).
-
Place the foil on the copper (ii) sulfate solution with
-
Observe and record observations. Be careful, do not touch!




3. sodium chloride and water
-
Measure 2.0g of sodium chloride and 25.0 ml water.
-
Mix water and sodium chloride to a beaker. Stir it until the salt is completely dissolved.
-
Prepare the burner set up as instructed by your instructor.
-
Heat up the solution until it completely evaporates. Caution: exercise care when handling hot objects
-
Observe and record observations.
4. ammonium nitrate and water
-
Measure the temperature of the distilled water.
-
Measure 2.0 g of ammonium nitrate with the scale and 25.0 ml water with graduated cylinder.
-
Add water to the ammonium nitrate.
-
Stir Completely and measure the temperature.
-
Observe and record observations.



first day!
observations and post-lab questions